High-level Research Publications with CIQTEK EPR Spectroscopy
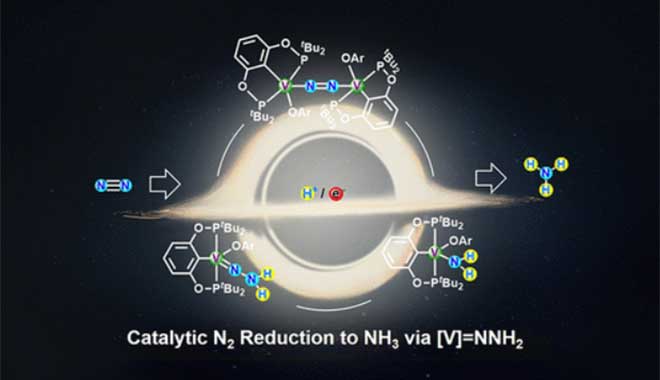
We are pleased to announce that the CIQTEK EPR spectroscopy has contributed to over 100 high-level research publications! (The list is shown below in Section #2) Section 1. One of the Selected Results Vanadium-Catalyzed Dinitrogen Reduction to Ammonia via a [V]═NNH2 Intermediate.Journal of the American Chemical Society (2023) Wenshuang Huang, Ling-Ya Peng, Jiayu Zhang, Chenrui Liu, Guoyong Song, Ji-Hu Su, Wei-Hai Fang, Ganglong Cui, and Shaowei Hu Abstract The Earth's atmosphere is rich in N2 (78%), but the activation and conversion of nitrogen have been challenging due to its chemical inertness. The ammonia industry uses high-temperature and high-pressure conditions to convert N2 and H2 to NH3 on the surface of solid catalysts. Under ambient conditions, certain microorganisms can bind and convert N2 to NH3 via Fe(Mo/V)-based nitrogen fixation enzymes. Although great progress has been made in the structure and intermediates of nitrogen fixation enzymes, the nature of N2 binding to the active site and the detailed mechanism of N2 reduction remains uncertain. Various studies on the activation of N2 with transition metal complexes have been carried out to better understand the reaction mechanism and to develop catalysts for ammonia synthesis under mild conditions. However, the catalytic conversion of N2 to NH3 by transition metal complexes remains challenging. Despite the crucial role of vanadium in biological nitrogen fixation, few well-defined vanadium complexes can catalyze the conversion of N2 to NH3. In particular, the V(NxHy) intermediates obtained from the proton/electron transfer reactions of ligated N2 remain unknown. Herein, this paper reports the vanadium metal complex-catalyzed reduction of nitrogen to ammonia and the first isolation and characterization of a neutral hydrazide complex intermediate ([V]=NNH2) from a nitrogen-activated system, with the cyclic conversion process simulated by the reduction of the protonated vanadium amino complex ([V]-NH2) to obtain a dinitrogen compound and release of ammonia. These findings provide unprecedented insights into the mechanism of N2 reduction associated with FeV nitrogen-fixing enzymes by combining theoretical calculations to elucidate the possible conversion of nitrogen to ammonia via the distal pathway in this catalytic system. The group of Prof. Dr. Shaowei Hu at Beijing Normal University is dedicated to the development of transition metal complexes for the activation of inert small molecules. Recently, in collaboration with Prof. Dr. Ganglong Cui's group, we reported the reduction of nitrogen to ammonia catalyzed by vanadium metal complexes through a combination of theoretical calculations and experimental studies. The results of this study were published in the Journal of the American Chemical Society, and Wenshang Huang (M.S. student) and Lingya Peng (Ph. D. student) were the co-first authors of t...